Charles Coffey, MD; Tamer Ghanem, MD, David Goldenberg, MD FACS and Ellie Maghami, MD, American Head & Neck Society Education Committee
Introduction
This document is intended to introduce a subtype of head and neck cancer known as oropharynx cancer. Normal anatomy and physiology of the oropharynx is reviewed. Factors that can lead to cancer formation in this area are defined. Information regarding diagnosis, treatment, and expected outcomes of this condition is provided.
Anatomy & Functional Considerations
The oropharynx is a critical functional component of the upper aerodigestive tract and is one of most common sites of origin of head and neck malignancy. For the purposes of functional and oncologic considerations it is important to distinguish the oropharynx from the oral cavity, which is a separate and distinct anatomic site. The oropharynx is comprised of the tonsils, base of tongue, soft palate, and pharyngeal walls. The soft palate is a muscular soft tissue sling which defines the superior extent of the oropharynx. It allows isolation of the nasopharynx from the remainder of the pharynx and oral cavity during speech and deglutition. Inability to close the soft palate (velopharyngeal insufficiency) due to tumor, resection, or fibrosis may result in hypernasal speech as well as nasopharyngeal reflux during swallowing. The palatine tonsils are collections of lymphoid tissue which reside on the lateral pharyngeal wall within a fossa formed by mucosal folds overlying the palatoglossus and palatopharyngeus muscles. Although the tonsils and associated lymphoid tissues of Waldeyer’s ring participate in humoral and cell-mediated immunity of the aerodigestive tract, removal of these tissues has not been demonstrated to compromise immune status (Böck ; Kaygusuz). The base of tongue comprises the ventral surface of the oropharynx, extending from the vallecula inferiorly to the circumvalate papillae anteriorly. Superficially lined by lymphoid tissue (lingual tonsils), the muscles of the base of tongue are critical in controlling passage of a food bolus during the pharyngeal phase of swallowing. Base of tongue dysfunction resulting from tumor, loss of tissue, denervation, or fibrosis may result in discoordination and delay in propulsion of food bolus, or premature spillage of liquids from the oral cavity into the pharynx and larynx. The later may result in aspiration. The lateral and posterior walls of the oropharynx are comprised primarily of the superior and middle constrictor muscles, which play a supporting role in the pharyngeal phase of swallowing.
Epidemiology
The oropharynx is one of the most common sites of head and neck cancer in the United States, currently trailing only the oral cavity in annual incidence among head and neck cancers (Siegel). The vast majority of these tumors are squamous cell carcinoma, an epithelial malignancy which may affect any tissue site lined with squamous epithelium. The incidence of oropharyngeal squamous cell carcinoma (OPSCC) in the U.S. is estimated at between 11,000-13,000 new cases per year (Jemal; Siegel; CDC). Males are more frequently affected, with an overall annual risk of 6.2 per 100,000 men compared to 1.4 cases per 100,000 women (Jemal, CDC).
The incidence of OPSCC has risen sharply over the last several decades, due primarily to increased rates of tumors associated with the human papilloma virus (HPV). Although the incidence of HPV-negative tumors in the U.S. declined by 50% from 1988-2004 (paralleling the decrease in smoking and smoking-related malignancies), the incidence of HPV-positive tumors increased by 225% during the same time frame (Chaturvedi). The proportion of oropharyngeal cancers in the U.S. which were HPV-positive increased from 16% in the period from 1984-89 to 72% in the period from 2000-04 (Chaturvedi). Similar trends have been seen elsewhere. Rates of OPSCC in Australia increased by 43% between 1982 and 2008 (Ariyawardana), and the incidence of tosillar cancer in Swedish men increased at a rate of 2.6% per year from 1960-2003 (Hammarstedt). The rate of HPV-positive tumors in Sweden nearly doubled each decade from 1970-2007 (Näsman). After steadily declining during the previous 3 decades, the age-adjusted mortality of oropharyngeal carcinoma in the U.S. has trended gradually upward since 2000 (0.38/100,000 deaths in 2000; 0.46/100,000 in 2010) (Jemal). This overall increase in mortality is a reflection of increased incidence of these tumors rather than a decrease in survival rates.
Historically, oropharyngeal carcinoma was primarily a disease of older individuals, with highest incidence over the age of 70 (Hammarsted). However, these demographics have shifted in the era of HPV-associated OPSCC. The last two decades have seen a steady increase in the rates of HPV-positive OPSCC among younger individuals, most notably white males between the ages of 50 and 70 (Chaturvedi, CDC).
Risk Factors
Oropharyngeal carcinoma rates are 4-fold higher in men than women across ethnicities. Incidence in the U.S. is highest among white and black men (age-adjusted incidence of 8.5/100,000 and 7.9/100,000, respectively), and lower among Hispanics (5.7/100,000 men), Native Americans (4.7/100,000 men), and Asians/ Pacific Islanders (2.2/100,000 men) (Siegel, Jemal).
Tobacco use has been strongly established as the primary carcinogenic factor in the majority of head and neck aerodigestive tract cancers (Sturgis 04, Sturgis 07). Lifetime risk of developing head and neck cancer is increased 10-fold in smokers, and the magnitude of risk increases in a dose-dependent fashion, up to 25-fold for the heaviest smokers. Alcohol use is an independent risk factor for development of head and neck cancer. The most significant risk is associated with chronic heavy alcohol use, though there is some evidence that light alcohol use may also increase risk of developing oropharyngeal cancer (Bagnardi). Though tobacco and alcohol themselves are independent risk factors, the risks associated with combined tobacco and alcohol use are synergistic (Masberg). Historically, up to 90% of head and neck squamous cell carcinoma including OPSCC has been attributed to tobacco use and alcohol abuse (Sturgis 04).
Tobacco use in the United States has been steadily declining for the last five decades. The percentage of active smokers in the U.S. decreased from 43% in 1965 to 21% in 2005 (Mariolis), and that number dropped below 20% in 2010 (Skinner). Per capita alcohol use has shown a more modest decline, from 2.7 gallons per year in the mid-1980s to 2.26 gallons per year in 2010 (LaVallee). These trends have generally been paralleled by decreasing rates in head and neck cancer incidence and mortality. However, as outlined in the previous section, rates of oropharyngeal cancer have trended steadily upward for the last decade, primarily due to the increase in HPV-related tumors. Since the first description of the association between HPV and head and neck cancer in 1983, the role of HPV in the development of squamous cell carcinoma has become well established (Syrjänen; Gillison 2004; Li). The primary molecular mechanism of HPV-related carcinogenesis in OPSCC is deactivation of tumor suppressor genes p53 and Rb by the viral oncogenes E6 and E7, respectively (Li; Rothenberg). A number of high-risk viral subtypes have been identified, most notably HPV-16, which is present in up to 95% of HPV-related oropharyngeal tumors (Gillison 2008).
The risk profile for HPV-related OPSCC differs from most head and neck cancers, supporting clinical and molecular evidence that this is a distinct pathologic entity (Gillison 2004). When compared to HPV-negative head and neck cancer patients, patients with HPV-positive OPSCC are more likely to be young, white, higher socioeconomic status, non-smokers, and non-drinkers. Sexual history is strongly associated with HPV-positive cancers. Significant increase in risk of OPSCC has been associated with lifetime number of sexual partners including oral sex partners, any history of oral sex, earlier age at sexual debut, infrequent use of barrier devices during sex, lifetime history of sexually transmitted disease, and, among men, with a history of same-sex sexual contact (Heck; Gillison 2008). The sexual risk factors associated with oral HPV infection mirror those seen with HPV-related OPSCC. Current prevalence of oral HPV infection in the U.S. among individuals aged 14 to 69 years is approximately 7%, while incidence of oncogenic (high risk) subtypes is 3% (Gillison 2012; Sanders). The time lag between an oral HPV infection and the development of HPV-related oropharyngeal cancer is estimated at between 15 and 30 years. As such, the rise in OPSCC seen since the 1990s may reflect changes in sexual practices in the 1960s and 1970s.
An association between head and neck cancer and marijuana use has been demonstrated by Gillison et al. (Gillison 2008). This association was limited to HPV-positive tumors, and although it is not clear that marijuana use is an independent risk factor for OPSCC, it is plausible that cannabinoids may promote progression of HPV-mediated carcinogenesis via immunologic mechanisms.
Poor oral health, including periodontal disease and tooth loss, has been implicated in the etiology of oral and oropharyngeal carcinoma, but the associations have been modest and the evidence has generally been inconclusive (Divaris). Recent evidence has demonstrated increased risk of oral HPV infection related to poor oral health and irrespective of smoking or oral sex history (Bui). This suggests that poor oral health may be an indirect risk factor for OPSCC, though this has not been borne out in other studies (Gillison 2008).
High dietary intake of fruit and vegetables may be somewhat protective for oral and pharyngeal cancer, though the evidence for this has not been conclusive, and at least part of the protective effect may be explained by confounding effects of tobacco and alcohol exposure (Lucenteforte).
Diagnosis & Evaluation
All patients with findings suspicious for oropharyngeal cancer should be referred to a head and neck surgeon or otolaryngologist for further evaluation. Initial evaluation consists of a detailed history and comprehensive head and neck examination, generally including flexible fiberoptic nasopharyngoscopy and laryngoscopy performed in an office setting. Any suspicious tumors of the oropharynx should be biopsied for histopathologic evaluation. In many instances it is possible to obtain an adequate tissue sample in clinic if a lesion is easily accessible either directly through the mouth or via flexible endoscopic biopsy. However, examination and biopsy under anesthesia may be required if achieving exposure for biopsy is more difficult; if medical considerations such as anticoagulation status preclude safe biopsy in an office setting; if airway intervention such as tracheostomy is required due to obstructive tumor; or if the clinic exam cannot provide sufficient evaluation of the primary tumor for accurate staging. In select circumstances, cytopathologic evaluation via fine needle aspiration of a metastatic neck node may prove adequate tissue to establish diagnosis, provided that the tumor can be accurately clinicaly staged without exam under anesthesia. Testing of specimens for HPV status is recommended in all cases, either through immunohistochemistry for p16 expression or in situ hybridization for detection of HPV DNA. Although HPV-status should not be used in treatment planning outside of a clinical trial setting, information regarding HPV-status is currently the single greatest prognostic factor in evaluation of OPSCC.
Once a diagnosis of OPSCC is confirmed, all patients should undergo multidisciplinary evaluation. Imaging should be obtained to evaluate the primary tumor, nodal basin, and chest. Computed tomography (CT) and magnetic resonance imaging (MRI) of the neck both allow appropriate diagnostic imaging of the pharynx and cervical nodal basins, and either modality may be selected based upon the preference of providers and patients. Either iodinated contrast (CT) or gadolinium (MRI) should be administered unless medically contraindicated. A diagnostic imaging study of the neck should extend from the skull base to the thoracic inlet, and will thus provide evaluation of both the pharynx and nodes with a single study. There is no routine role for imaging of the head or brain in the evaluation of oropharyngeal cancer. Imaging of the chest generally consists of diagnostic-quality chest CT, although PET/CT may be considered in evaluation of advanced-stage disease (Stage III-IV) due to increased risk of distant metastasis.
Patients considered for radiation therapy should undergo dental evaluation prior to treatment, due to the increased risk of mandibular osteoradionecrosis associated with dental procedures (extraction, e.g.) when performed following radiation. If there is evidence of dental decay or advanced periodontal disease, prophylactic extraction prior to undergoing radiation is frequently recommended. Pretreatment nutrition and speech and swallowing evaluations should be provided to all patients. Patients experiencing significant weight loss or swallowing difficulty may benefit from feeding tube placement prior to treatment, although many patients are able to maintain an oral diet throughout the duration of treatment with either surgery-based or radiation-based approaches.
Staging
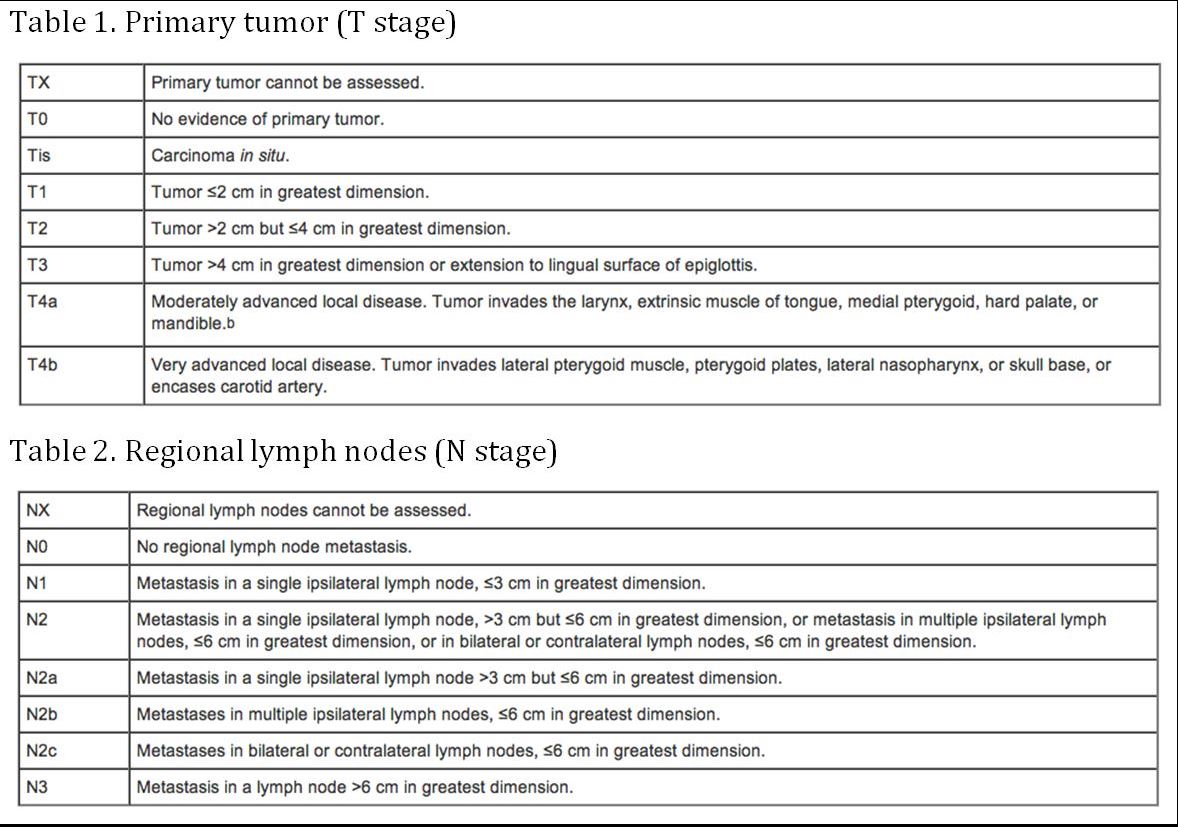
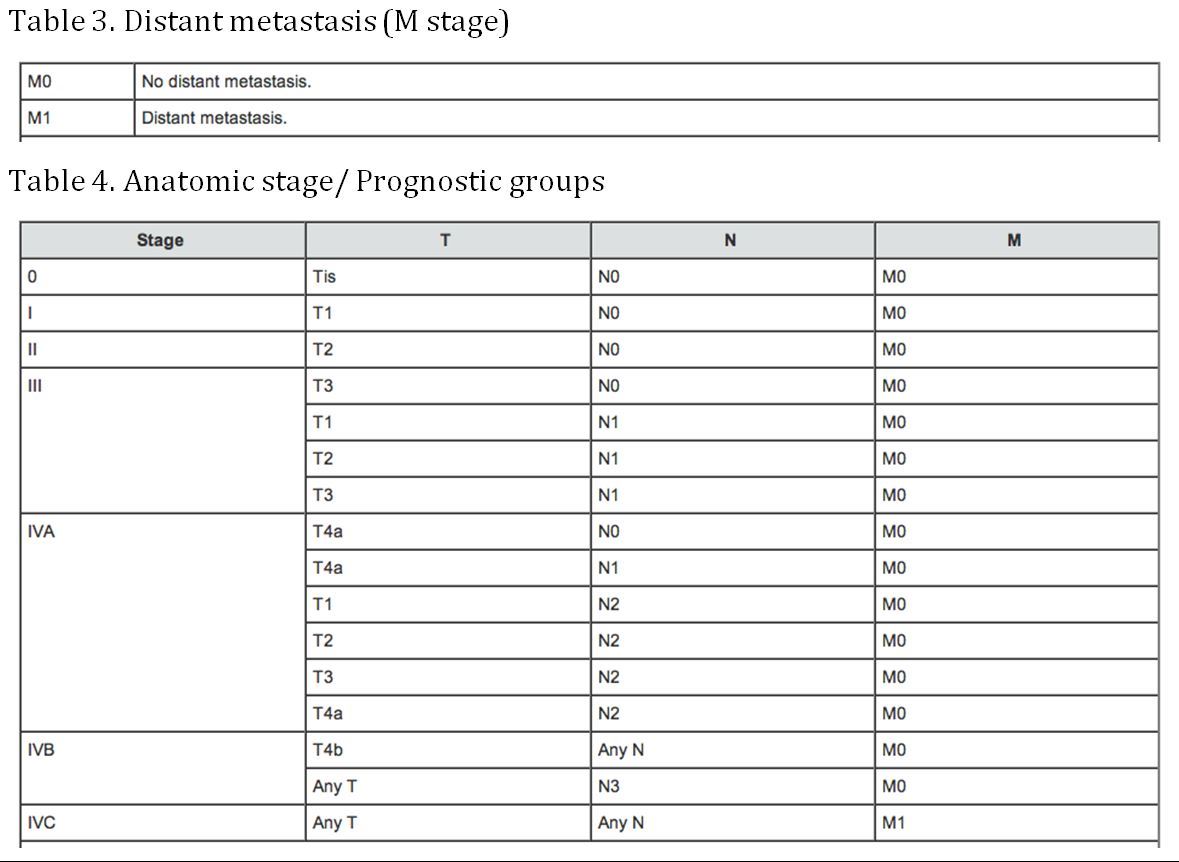
(AJCC: Pharynx. In: Edge SB, Byrd DR, Compton CC, et al., eds.: AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer, 2010, pp 41-56)
Treatment
Treatment paradigms
Current NCCN guidelines for management of OPSCC may be broadly divided based upon the distinction between early-stage (I-II) and advanced-stage (III-IV) disease. Early-stage disease may generally be treated with single-modality therapy, consisting of either surgery or definitive radiation. Either modality should include management of both the primary tumor site within the oropharynx and also the at-risk cervical nodal basin. Treatment of the cervical nodes is required even for early-stage (clinically N0) disease due to the elevated risk for occult nodal metastasis associated with tumors of the oropharynx (Byers; Shah). If surgery is employed for early-stage disease, the addition of pathologic staging information helps guide whether additional treatments are warranted. For example, adjuvant radiation therapy (also referred to as postoperative radiation therapy, or PORT) may be recommended for select T1 and T2 disease when histopathologic evaluation demonstrates intermediate-risk adverse features such as perineural invasion, multiple metastatic lymph nodes, or close surgical margins. Adjuvant chemotherapy may be employed when high-risk adverse features are identified, included the presence of extracapsular nodal disease or positive surgical margins(Cooper, Pajak et al. 2004) .
Advanced-stage tumors of the oropharynx require multimodality therapy. This may include primary surgery followed by adjuvant radiotherapy, or concurrent chemoradiation. Alternatively, induction chemotherapy may be employed prior to definitive radiotherapy or chemoradiation in an attempt to minimize risk of distant metastasis in patients considered to be at high-risk due to extensive primary tumor or high-volume nodal disease (Posner and Vermorken 2008, Vermorken 2010). In either setting, the primary effect of cytotoxic chemotherapy is to serve as a radiosensitizer. If residual tumor remains either at the primary site or within the neck following completion of definitive chemoradiation, surgery may be employed (Zafereo, Hanasono et al. 2009, Zafereo 2014) in an attempt to eradicate residual or recurrent disease. However, long-term outcomes for salvage surgery following failure of primary chemoradiation for OPSCC are often poor .
The current standard-of-care for administration of radiation to the orpharynx is intensity modulated radiation treatment (IMRT). This technique utilizes sophisticated imaging to construct a three-dimensional map defining the treatment dose to the tumor and surrounding tissues. The advantage of IMRT over older techniques is the ability to optimize radiation delivery to the tumor site while minimizing dose to surrounding critical structures such as the spine, carotid arteries, and brain, and increasingly to spare portions of functionally critical structures such as the salivary glands and superior constrictor muscles . The efficacy of IMRT over conventional radiation for treatment of oropharyngeal cancer has yet to be well established. There are several dose-dependent side effects of head & neck radiation. Patients commonly complain of xerostomia, loss of taste, dysphagia (in some cases require feeding tube placement), fibrosis of neck skin and muscles, and oral and oropharyngeal mucositis. Late side effects include increased risk of carotid atherosclerotic disease, esophageal stricture, osteoradionecrosis of the mandible, and the possibility of a radiation induced malignancy.
Surgical approaches
Surgery of oropharyngeal tumors can be performed through various approaches, to obtain access to the oropharynx dictated by the location and size of the tumor as well as functional and aesthetic concerns. Traditional “open” approaches to the oropharynx include a transmandibular approach via lip-splitting incision and mandibulotomy or transcervical approaches involving neck dissection and incisions into the anterior or lateral pharynx (Coffey; Mehta). These approaches offer excellent surgical exposure of the tonsillar fossa, soft palate, and base of tongue, though they are often accompanied by significant functional morbidity due to the extensive dissection required. The majority of these patients require short-term tracheostomy and/ or feeding tube placement, and soft tissue reconstruction with a vascularized flap is often require to close the surgical defect and reduce the risk of fistula formation. Reconstruction frequently consists of regional flaps such as the pectoralis major flap or various microvascular flaps, each of which is accompanied by additional morbidities. Additional disadvantages of these open approaches includes prolonged operative times, significant postoperative edema, external scarring, and the potential for mandibular complications including malunion, nonunion or bite abnormality. Given the increasing use and proven efficacy of radiation therapy, most centers in the United States currently reserve open surgical approaches for salvage cases where additional radiation is not possible or too risky, and where less invasive surgical approaches could compromise oncologic outcomes.
The last several decades have seen the advent of transoral surgical approaches for management of OPSCC. Although very early stage lesions of the tonsil or base of tongue may be resectable with very basic equipment and minimal technical difficulty, the challenging anatomy and difficult exposure of the oropharynx often preclude such straightforward approaches. However, recent technical advances have allowed surgeons to resect even relatively large tumors of the oropharynx via strictly transoral approaches, most notably including transoral laser microsurgery (TLM), or transoral robotic surgery (TORS). Transoral laser microsurgery was popularized by Steiner (representative citations), and combines conventional suspension laryngoscopy techniques with microscopic magnification and CO2 laser to remove tumors of the oropharynx and larynx. The advantages of TLM include improved access and visualization in difficult areas such as suprglottis and glottis, and relative availability of the required instrumentation. Disadvantages of TLM include a steep learning curve and the limitations of line-of-sight laser application.
In December of 2010, TORS was approved by the FDA for management of T1 and T2 oropharyngeal and supraglottic cancers, and this approach is gaining increasing popularity in the U.S. and abroad. TORS capitalizes on the abilities conferred by the Da Vinci Robot (Intuitive Surgical), including three-dimensional visualization of the target anatomy and the ability to work precisely in a confined space such as the oropharynx. The TORS surgeon sits on a console allowing control the movement of robotic arms, which are introduced to the surgical site through the mouth with the aid of a retractor to obtain exposure of the oropharynx. The primary advantages of this approach include more rapid learning curve than TLM, ability to resect tumor en bloc instead of piecemeal, excellent three-dimensional magnified view of the relevant anatomy, and precise articulated instrumentation. The disadvantages include limited availability due to need for expensive surgical equipment and specialty training of the surgical and OR staff. Numerous studies on the oncological efficacy of this approach have been published, demonstrating excellent oncologic control equivalent to or better than standard surgical and non-surgical techniques (References- consider White 2013; de Almeida 2014; others)).
The general advantages of transoral surgical approaches include decreased morbidity compared with more extensive open dissections, avoidance of tracheostomy, decreased blood loss, and shorter surgical times. (Include data/ citations regarding specific functional outcomes; consider More 2013). Decreased hospital stays are often possible as well (White 2013). However, due to anatomic and technical constraints not all tumors are amenable to transoral resection, and these techniques also require considerable experience on the part of the surgeon, as well as instrumentation which may not be available at many centers.
Follow up & Outcomes
Nonsurgical Treatment
One of the difficulties in comparing outcomes between surgical and non-surgical treatments for oropharyngeal cancer is lack of prospective randomized control trials to assess the efficacy and functional outcomes of the different available treatments. This is an area currently being investigated through RTOG and ECOG trials. However, the trend in the past 10-15 years has been use of concomitant chemoradiation therapy. There are several studies that have evaluated the response of combined radiation and chemotherapy on oropharyngeal cancer. In a large recently published single-institution study, 1046 patients with oropharygneal cancer were treated at a single institution with non-surgical treatment (radiation alone, concomitant chemoradiation, or induction chemo followed by chemoradiation) 5-year year overall survival, local control, regional control rates were 78%, 77%, and 87%, respectively (Garden). An older, yet highly cited study, performed a meta-analysis of 51 published studies on oropharyngeal cancer from 1970-2000 (Parsons). They found that the rates of cancer control between surgery followed by radiation therapy versus radiation therapy alone were similar: local control 79% versus 76%, loco-regional control 60% versus 69%, 57% versus 59% 5-year disease specific survival , respectively. While the cancer control was not significantly different between the cohorts, the rates of severe complications were much higher in the surgery group versus the primary radiation group, 23% vs. 6%. Similarly the rates of fatal complications were higher in the surgery group versus the radiation group 3.2% versus 0.8%, respectively. The primary drawback of this study is the inclusion of older literature, which favored open surgical approaches with and without reconstructive techniques, and pre-modern radiation techniques such as intensity modulated radiotherapy therapy (IMRT). Furthermore, newer literature supports adding chemotherapy to radiation to improve cancer control rates (Blanchard; Pignon 2007; Pignon 2009). Unfortunately, the addition of chemotherapy to radiation therapy increases the toxicity profile of the treatment regimen, and can severely affect the patient’s quality of life especially in the areas of deglutition and xerostomia.
Surgical Treatment
Using open surgical approaches for base of tongue cancer ablation followed by adjuvant treatment, results are variable depending on T-stage of the disease. In a study from Pittsburgh with 87 patients with base of tongue cancer, overall and disease specific survival at 5-years were 49% and 56%, respectively (Gourin and Johnson 2001). For 5-year disease specific survival T1 was 88%, T2 was 64%, T3 was 58%, and T4 was 30%. Utilizing transoral surgical approaches, whether TLM or TORS, excellent tumor control can be achieved as reported by multiple investigators. White and colleagues reported an 86.5% recurrence free survival at two years follow up in a cohort of 89 patients (T1, T2 n=79; T3, T4=18) (White, Moore et al. 2010). In a separate group of 47 patients, treated TORS by Weinstein, disease specific survival was 90% at 2 years. In this cohort, 38% avoided additional chemotherapy as a result of undergoing surgical treatment, and 11% of patients did not require adjuvant radiotherapy. Also, one of out the 47 patients require gastrostomy tube placement at one year. Similarly, the data in the TLM literature is very robust. In a recent study by Patel and colleagues from Mayo Clinic, the locoregional control , recurrence free and overall survival rates at 3 years in a cohort of 80 patients (T1 52.5%, T2 38.8%, and T3 8.8%) were 98.6%, 91.1%, and 93.7%, respectively.
References
Skinner HD, Holsinger FC, Beadle BM. Oropharynx cancer. Curr Probl Cancer 2012; 36:334-415
Coffey CS, Day TA. (2013) Cancer of the oral cavity: surgical technique. In Head and Neck Cancer: A Multidisciplinary Approach, 4th edition. Eds. Harrison LB, Sessions RB, Kies MS. Philadephia, PA: Lippincott, Williams, and Wilkins
Mehta V, Ferris RL. (2013) Cancer of the oropharynx: surgical technique. In Head and Neck Cancer: A Multidisciplinary Approach, 4th edition. Eds. Harrison LB, Sessions RB, Kies MS. Philadephia, PA: Lippincott, Williams, and Wilkins
