Diana N Kirke MBBS MPhil FRACS, Richard Torbeck MD
There are, according to the American Cancer Society, approximately 5.4 million basal (BCC) and cutaneous squamous cell carcinomas (cSCC) diagnosed each year in the US, with 80% of these being BCC.1 As head and neck surgeons we tend to see more advanced presentations of cutaneous malignancy. However, it is important to appreciate all the treatment options for early forms of BCC and cSCC. It is especially prudent because the patient may have been treated with topical therapies prior to them presenting in our office.
Like all head and neck malignancies, a multidisciplinary approach is vital. The realm of cutaneous malignancy extends past radiation and medical oncologists to include the dermatologist. Dermatologists play a central role by conducting regular skin surveillance for skin cancer patients, prescribing topical treatments for early cutaneous malignancy and/or providing Mohs micrographic surgery (MMS) as required. All of this provides an opportunity for collaboration in terms of both patient management and research endeavors.
After biopsy confirms the diagnosis of a cutaneous malignancy, surgery is considered the gold standard of treatment. However, in certain indications, topical treatments for both BCC and cSCC can be considered to help balance the need for both tumor removal and a good cosmetic result. In general terms, small and superficial BCCs can be treated with topical agents, while for cSCC they are generally reserved for cSCC in-situ (cSCCIS).
There are obvious advantages and disadvantages to their use. In general, a clear advantage is the possibility of less invasive and superior cosmetic outcomes. A very clear disadvantage is the lack of pathologic confirmation of complete tumor removal. More specifically to this, the patient needs to be made aware that the cure rates are lower for topical treatments than they are for surgical treatment modalities.2,3
The topical treatments for BCC and cSCC are similar and include fluorouracil (5-FU – EfudexÒ, CaracÒ, FluoroplexÒ, TolakÒ and Imiquimod (AldaraÒ, ZyclaraÒ).
5-FU is an FDA approved therapy for superficial BCC.2 For cSCC topical 5-FU may play a role in either multiple superficial (that is, non-invasive) lesions, those that refuse surgical treatment or in cSCCIS. It works by inhibiting cell proliferation by interfering with DNA and RNA synthesis. Specifically, it prevents the enzyme thymidylate synthetase. It is applied topically as a 5% cream/solution and is applied twice daily for 4 to 6 weeks. Erythema, desquamation, and crusting are to be expected.
Imiquimod is also an FDA approved topical treatment for superficial BCC. It has also been shown to be effective in those with cSCCIS where high rates of clearance and low rates of recurrence have been reported.3 It works by upregulating the immune response via interferons and other cytokines. Usage on lesions less than 2cm in diameter results in a histological clearance rate of 82%. It is applied topically once daily five days per week for 6-12 weeks. Mild to moderate skin irritation is the aim of treatment and the dose can be titrated until this is achieved.
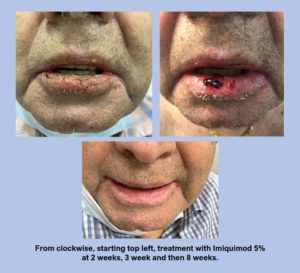
The photoseries below (see Figure 1) demonstrates the use of Imiquimod in an immunosuppressed patient with cSCCIS involving the right lower lip which was biopsy proven. Patient presented with a vague indistinct red and crusted lower lip. After prolonged discussion Imiquimod 5% for five nights a week for 6 weeks was initiated. Patient had prominent crusting blistering and pain that led to two short periods of drug discontinuation. After reassurance and evaluation the patient eventually completed the entire 6-week course after 8 weeks. Patient is in clinically evident remission at this time.
 Newer options to topical therapies for precancerous lesions on the skin are Picato® (Igenol mebutate) and Klisyri® (Tirbanibulin). Picato® is a topical that comes in two formulations, one for the face (0.015% for two days) and one for the trunk (0.05% for three days). In the initial trials 42% demonstrated complete clearance with minimal side effects. Klisyri® is applied for five days to the face for actinic keratosis. Clearance rate in their initial studies demonstrated 44% (77/175) complete clearance with few side effects.4,5 These treatments are also currently being explored in for cSCC.
Newer options to topical therapies for precancerous lesions on the skin are Picato® (Igenol mebutate) and Klisyri® (Tirbanibulin). Picato® is a topical that comes in two formulations, one for the face (0.015% for two days) and one for the trunk (0.05% for three days). In the initial trials 42% demonstrated complete clearance with minimal side effects. Klisyri® is applied for five days to the face for actinic keratosis. Clearance rate in their initial studies demonstrated 44% (77/175) complete clearance with few side effects.4,5 These treatments are also currently being explored in for cSCC.
If not currently a prescriber of these topical treatments, one should work collaboratively with their dermatological colleagues to appropriately treat superficial BCC and cSCCIS. Working in this manner will not only keep one abreast of emerging topical treatments, but it will also better inform all aspects of the patients’ cutaneous malignancy management and ultimately improve their long-term care.
References
- Key statistics for basal and squamous cell skin cancers. American Cancer Society. Available from http://www.cancer.org/cancer/skincancer-basalandsquamouscell/detailedguide/skin-cancer-basal-and-squamous-cell-key-statistics. January 8, 2020; Accessed: February 1, 2020.
- National Comprehensive Cancer Network. Basal Cell Skin Cancer Version 1.2022, 11/17/21. Available from https://www.nccn.org/professionals/physician_gls/pdf/nmsc.pdf; Accessed: December 6, 2021.
- National Comprehensive Cancer Network. Squamous Cell Skin Cancer Version 1.2022, 11/17/21. Available from https://www.nccn.org/professionals/physician_gls/pdf/squamous.pdf; Accessed: December 6, 2021.
- Lebwohl M, Shumack S, Gold LS, Melgaard A, Larsson T, Tyring SK. Long-term Follow-up Study of Ingenol Mebutate Gel for the Treatment of Actinic Keratoses. JAMA Dermatol. 2013;149(6):666–670.
- Blauvelt A, Kempers S, Lain E, Schlesinger T, Tyring S, Forman S, Ablon G, Martin G, Wang H, Cutler DL, Fang J, Kwan MR; Phase 3 Tirbanibulin for Actinic Keratosis Group. Phase 3 Trials of Tirbanibulin Ointment for Actinic Keratosis. N Engl J Med. 2021 Feb 11;384(6):512-520.
 Diana N. Kirke, MD, is an Assistant Professor in the Department of Otolaryngology- Head and Neck Surgery at Mount Sinai Doctors Faculty Practice, located at 5 East 98th Street. She received her medical degree from the University of Queensland and completed her Otolaryngology Residency via the Royal Australasian College of Surgeons in Adelaide, Australia. After completing her training, she relocated to New York to complete a Research Fellowship, followed by a Head and Neck Microvascular Fellowship at Boston University, and then a Laryngology Fellowship back in New York. Given her Australian upbringing she has an interest in cutaneous malignancy.
Diana N. Kirke, MD, is an Assistant Professor in the Department of Otolaryngology- Head and Neck Surgery at Mount Sinai Doctors Faculty Practice, located at 5 East 98th Street. She received her medical degree from the University of Queensland and completed her Otolaryngology Residency via the Royal Australasian College of Surgeons in Adelaide, Australia. After completing her training, she relocated to New York to complete a Research Fellowship, followed by a Head and Neck Microvascular Fellowship at Boston University, and then a Laryngology Fellowship back in New York. Given her Australian upbringing she has an interest in cutaneous malignancy.
 Richard L. Torbeck III, MD has an appointment in the rank of Assistant Professor in the Kimberly and Eric J. Waldman Department of Dermatology at The Icahn School of Medicine at Mount Sinai and Director of Skin Cancer Surgery Blavatnick Family Chelsea Medical Center. In 2014, Dr. Torbeck began his dermatology training at Thomas Jefferson University Department of Dermatology & Cutaneous Biology. Immediately following residency, Dr. Torbeck returned to the Icahn School of Medicine for a ACGME fellowship in Mohs Micrographic Surgery and Dermatologic Oncology and ASDS Cosmetic Dermatologic Surgery Fellowship.
Richard L. Torbeck III, MD has an appointment in the rank of Assistant Professor in the Kimberly and Eric J. Waldman Department of Dermatology at The Icahn School of Medicine at Mount Sinai and Director of Skin Cancer Surgery Blavatnick Family Chelsea Medical Center. In 2014, Dr. Torbeck began his dermatology training at Thomas Jefferson University Department of Dermatology & Cutaneous Biology. Immediately following residency, Dr. Torbeck returned to the Icahn School of Medicine for a ACGME fellowship in Mohs Micrographic Surgery and Dermatologic Oncology and ASDS Cosmetic Dermatologic Surgery Fellowship.

 Register for the AHNS 2022 Annual Meeting & Attend the PI Meeting for Clinical Trial NRG- HN006
Register for the AHNS 2022 Annual Meeting & Attend the PI Meeting for Clinical Trial NRG- HN006
Questions and Comments